Lunde S, Kristoffersen EK, Sapkota D, Risa K, Dahl O, Bruland O, et al. (2016) Serum BAFF and APRIL Levels, T-Lymphocyte Subsets, and Immunoglobulins after B-Cell Depletion Using the Monoclonal Anti-CD20 Antibody Rituximab in Myalgic Encephalopathy/Chronic Fatigue Syndrome. PLoS ONE 11(8): e0161226. doi:10.1371/journal.pone.0161226
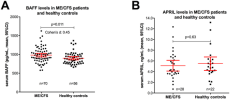
Myalgic Encephalopathy/Chronic Fatigue Syndrome (ME/CFS) is a disease of unknown etiology. We have previously suggested clinical benefit from B-cell depletion using the monoclonal anti-CD20 antibody rituximab in a randomized and placebo-controlled study. Prolonged responses were then demonstrated in an open-label phase-II study with maintenance rituximab treatment. Using blood samples from patients in the previous two clinical trials, we investigated quantitative changes in T-lymphocyte subsets, in immunoglobulins, and in serum levels of two B-cell regulating cytokines during follow-up. B-lymphocyte activating factor of the tumor necrosis family (BAFF) in baseline serum samples was elevated in 70 ME/CFS patients as compared to 56 healthy controls (p = 0.011). There were no significant differences in baseline serum BAFF levels between patients with mild, moderate, or severe ME/CFS, or between responders and non-responders to rituximab. A proliferation-inducing ligand (APRIL) serum levels were not significantly different in ME/CFS patients compared to healthy controls at baseline, and no changes in serum levels were seen during follow-up. Immunophenotyping of peripheral blood T-lymphocyte subsets and T-cell activation markers at multiple time points during follow-up showed no significant differences over time, between rituximab and placebo groups, or between responders and non-responders to rituximab. Baseline serum IgG levels were significantly lower in patients with subsequent response after rituximab therapy compared to non-responders (p = 0.03). In the maintenance study, slight but significant reductions in mean serum immunoglobulin levels were observed at 24 months compared to baseline; IgG 10.6–9.5 g/L, IgA 1.8–1.5 g/L, and IgM 0.97–0.70 g/L. Although no functional assays were performed, the lack of significant associations of T- and NK-cell subset numbers with B-cell depletion, as well as the lack of associations to clinical responses, suggest that B-cell regulatory effects on T-cell or NK-cell subsets are not the main mechanisms for the observed improvements in ME/CFS symptoms observed in the two previous trials. The modest increase in serum BAFF levels at baseline may indicate an activated B-lymphocyte system in a subgroup of ME/CFS patients.

 journals.plos.org
journals.plos.org
I don’t think this article was ever posted on here with its own thread.
Was there ever follow up with BAFF finding?
Myalgic Encephalopathy/Chronic Fatigue Syndrome (ME/CFS) is a disease of unknown etiology. We have previously suggested clinical benefit from B-cell depletion using the monoclonal anti-CD20 antibody rituximab in a randomized and placebo-controlled study. Prolonged responses were then demonstrated in an open-label phase-II study with maintenance rituximab treatment. Using blood samples from patients in the previous two clinical trials, we investigated quantitative changes in T-lymphocyte subsets, in immunoglobulins, and in serum levels of two B-cell regulating cytokines during follow-up. B-lymphocyte activating factor of the tumor necrosis family (BAFF) in baseline serum samples was elevated in 70 ME/CFS patients as compared to 56 healthy controls (p = 0.011). There were no significant differences in baseline serum BAFF levels between patients with mild, moderate, or severe ME/CFS, or between responders and non-responders to rituximab. A proliferation-inducing ligand (APRIL) serum levels were not significantly different in ME/CFS patients compared to healthy controls at baseline, and no changes in serum levels were seen during follow-up. Immunophenotyping of peripheral blood T-lymphocyte subsets and T-cell activation markers at multiple time points during follow-up showed no significant differences over time, between rituximab and placebo groups, or between responders and non-responders to rituximab. Baseline serum IgG levels were significantly lower in patients with subsequent response after rituximab therapy compared to non-responders (p = 0.03). In the maintenance study, slight but significant reductions in mean serum immunoglobulin levels were observed at 24 months compared to baseline; IgG 10.6–9.5 g/L, IgA 1.8–1.5 g/L, and IgM 0.97–0.70 g/L. Although no functional assays were performed, the lack of significant associations of T- and NK-cell subset numbers with B-cell depletion, as well as the lack of associations to clinical responses, suggest that B-cell regulatory effects on T-cell or NK-cell subsets are not the main mechanisms for the observed improvements in ME/CFS symptoms observed in the two previous trials. The modest increase in serum BAFF levels at baseline may indicate an activated B-lymphocyte system in a subgroup of ME/CFS patients.
Serum BAFF and APRIL Levels, T-Lymphocyte Subsets, and Immunoglobulins after B-Cell Depletion Using the Monoclonal Anti-CD20 Antibody Rituximab in Myalgic Encephalopathy/Chronic Fatigue Syndrome
Myalgic Encephalopathy/Chronic Fatigue Syndrome (ME/CFS) is a disease of unknown etiology. We have previously suggested clinical benefit from B-cell depletion using the monoclonal anti-CD20 antibody rituximab in a randomized and placebo-controlled study. Prolonged responses were then...
I don’t think this article was ever posted on here with its own thread.
Was there ever follow up with BAFF finding?
Last edited: