SARS-CoV-2 variants mediated tissue-specific metabolic reprogramming determines the disease pathophysiology in a hamster model
Kaur Sardarni; Ambikan; Acharya; Johnson; Avedissian; Végvári; Neogi; Byrareddy
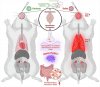
Despite significant effort, a clear understanding of host tissue-specific responses and their implications for immunopathogenicity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant infection has remained poorly defined.
To shed light on the interaction between tissues and SARS-CoV-2 variants, we sought to characterize the complex relationship among acute multisystem manifestations, dysbiosis of the gut microbiota, and the resulting implications for SARS-CoV-2 variant-specific immunopathogenesis in the Golden Syrian Hamster (GSH) model using multi-omics approaches.
Our investigation revealed the presence of increased SARS-CoV-2 genomic RNA in diverse tissues of delta-infected GSH compared to the omicron variant. Multi-omics analyses uncovered distinctive metabolic responses between the delta and omicron variants, with the former demonstrating dysregulation in synaptic transmission proteins associated with neurocognitive disorders. Additionally, delta-infected GSH exhibited an altered fecal microbiota composition, marked by increased inflammation-associated taxa and reduced commensal bacteria compared to the omicron variant.
These findings underscore the SARS-CoV-2-mediated tissue insult, characterized by modified host metabolites, neurological protein dysregulation, and gut dysbiosis, highlighting the compromised gut-lung-brain axis during acute infection.
Link | PDF (Brain, Behavior, and Immunity)
Kaur Sardarni; Ambikan; Acharya; Johnson; Avedissian; Végvári; Neogi; Byrareddy
Despite significant effort, a clear understanding of host tissue-specific responses and their implications for immunopathogenicity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant infection has remained poorly defined.
To shed light on the interaction between tissues and SARS-CoV-2 variants, we sought to characterize the complex relationship among acute multisystem manifestations, dysbiosis of the gut microbiota, and the resulting implications for SARS-CoV-2 variant-specific immunopathogenesis in the Golden Syrian Hamster (GSH) model using multi-omics approaches.
Our investigation revealed the presence of increased SARS-CoV-2 genomic RNA in diverse tissues of delta-infected GSH compared to the omicron variant. Multi-omics analyses uncovered distinctive metabolic responses between the delta and omicron variants, with the former demonstrating dysregulation in synaptic transmission proteins associated with neurocognitive disorders. Additionally, delta-infected GSH exhibited an altered fecal microbiota composition, marked by increased inflammation-associated taxa and reduced commensal bacteria compared to the omicron variant.
These findings underscore the SARS-CoV-2-mediated tissue insult, characterized by modified host metabolites, neurological protein dysregulation, and gut dysbiosis, highlighting the compromised gut-lung-brain axis during acute infection.
Link | PDF (Brain, Behavior, and Immunity)
Last edited: