Andy
Senior Member (Voting rights)
Preprint
Abstract
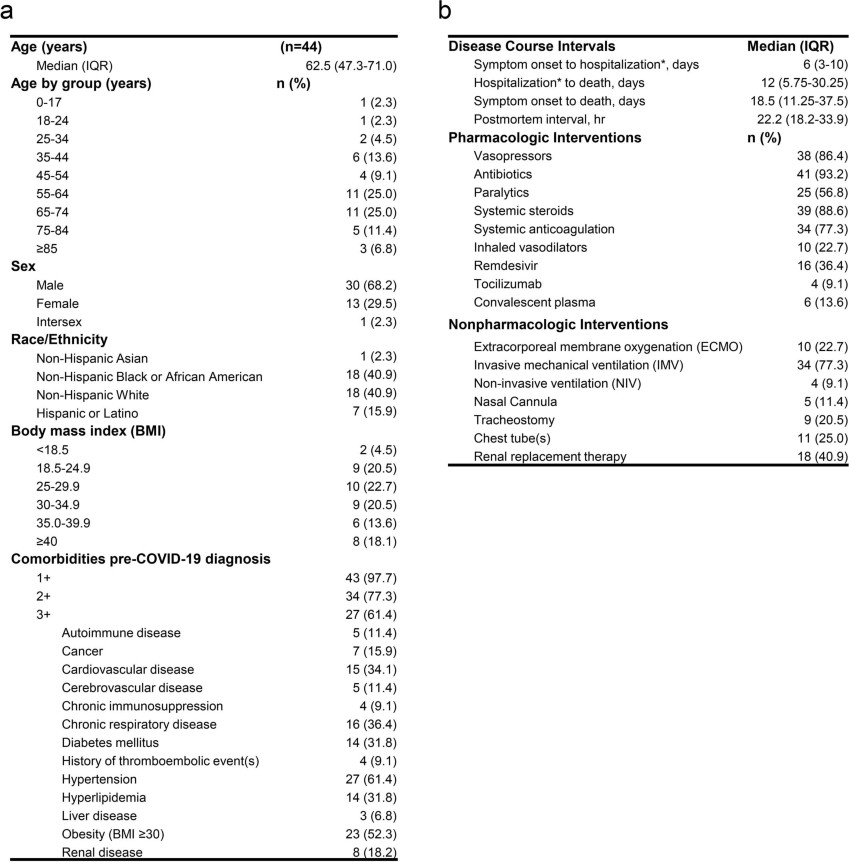
COVID-19 is known to cause multi-organ dysfunction1-3 in acute infection, with prolonged symptoms experienced by some patients, termed Post-Acute Sequelae of SARS-CoV-2 (PASC)4-5. However, the burden of infection outside the respiratory tract and time to viral clearance is not well characterized, particularly in the brain3,6-14. We performed complete autopsies on 44 patients with COVID-19 to map and quantify SARS-CoV-2 distribution, replication, and cell-type specificity across the human body, including brain, from acute infection through over seven months following symptom onset. We show that SARS-CoV-2 is widely distributed, even among patients who died with asymptomatic to mild COVID-19, and that virus replication is present in multiple extrapulmonary tissues early in infection. Further, we detected SARS-CoV-2 RNA in multiple anatomic sites, including regions throughout the brain, for up to 230 days following symptom onset. Despite extensive distribution of SARS-CoV-2 in the body, we observed a paucity of inflammation or direct viral cytopathology outside of the lungs. Our data prove that SARS-CoV-2 causes systemic infection and can persist in the body for months.
Open access, https://www.researchsquare.com/article/rs-1139035/v1
Abstract
COVID-19 is known to cause multi-organ dysfunction1-3 in acute infection, with prolonged symptoms experienced by some patients, termed Post-Acute Sequelae of SARS-CoV-2 (PASC)4-5. However, the burden of infection outside the respiratory tract and time to viral clearance is not well characterized, particularly in the brain3,6-14. We performed complete autopsies on 44 patients with COVID-19 to map and quantify SARS-CoV-2 distribution, replication, and cell-type specificity across the human body, including brain, from acute infection through over seven months following symptom onset. We show that SARS-CoV-2 is widely distributed, even among patients who died with asymptomatic to mild COVID-19, and that virus replication is present in multiple extrapulmonary tissues early in infection. Further, we detected SARS-CoV-2 RNA in multiple anatomic sites, including regions throughout the brain, for up to 230 days following symptom onset. Despite extensive distribution of SARS-CoV-2 in the body, we observed a paucity of inflammation or direct viral cytopathology outside of the lungs. Our data prove that SARS-CoV-2 causes systemic infection and can persist in the body for months.
Open access, https://www.researchsquare.com/article/rs-1139035/v1
Last edited by a moderator: