pooriepoor91
Established Member
Safety, tolerability and clinical effects of BC007 on fatigue and quality of life in patients with post-COVID syndrome (reCOVer): a prospective, exploratory, randomised , placebo-controlled, double-blind, crossover phase IIa clinical trial
Bettina Hohberger, Marion Ganslmayer, Thomas Harrer, Friedrich Kruse, Stefanie Maas, Tobias Borst, Ralph Heimke-Brinck, Andreas Stog, Thomas Knauer, Eva Ruehl, Victoria Zeisberg, Adam Skornia, Alexander Bartsch, Armin Stroebel, Monika Wytopil, Carolin Merkel, Sophia Hofmann, Katja G. Schmidt, Petra Lakatos, Julia Schottenhamml, Martin Herrmann, Christian Mardin, Juergen Rech
Abstract
Background: As recent data suggest an involvement of GPCR-fAAb in PCS pathogenesis, neutralisation of such GPCR-fAAbs by BC007 could improve PCS symptoms. The aim of the reCOVer trial was to investigate safety, tolerability and clinical effects of BC007 on fatigue, its severity and quality of life in PCS patients.
Methods: reCOVer is a prospective, exploratory, randomized, placebo-controlled, double-blind, crossover phase IIa clinical trial with 1350 mg BC007 at the University of Erlangen-Nuernberg, Germany. Eligible participants were 18-80 years with GPCR-fAAb, whose PCS symptoms persisted ≥3 months after PCR-confirmed COVID-19, with fatigue as the major symptom (Bell score ≤60) and at least three of eight defined PCS symptoms. Participants were randomly assigned (1:1) according to a crossover design to either receive BC007 (sequence A) or placebo (sequence B) at day 0 and day 48 with a follow-up of 28 days, respectively. A crossover design was chosen to increase patient adherence. Occurrence of treatment-emergent adverse events (TEAEs) in comparison between sequence A and B from d0 to d28 and d0 to d70 were the primary and co-primary endpoint, respectively.
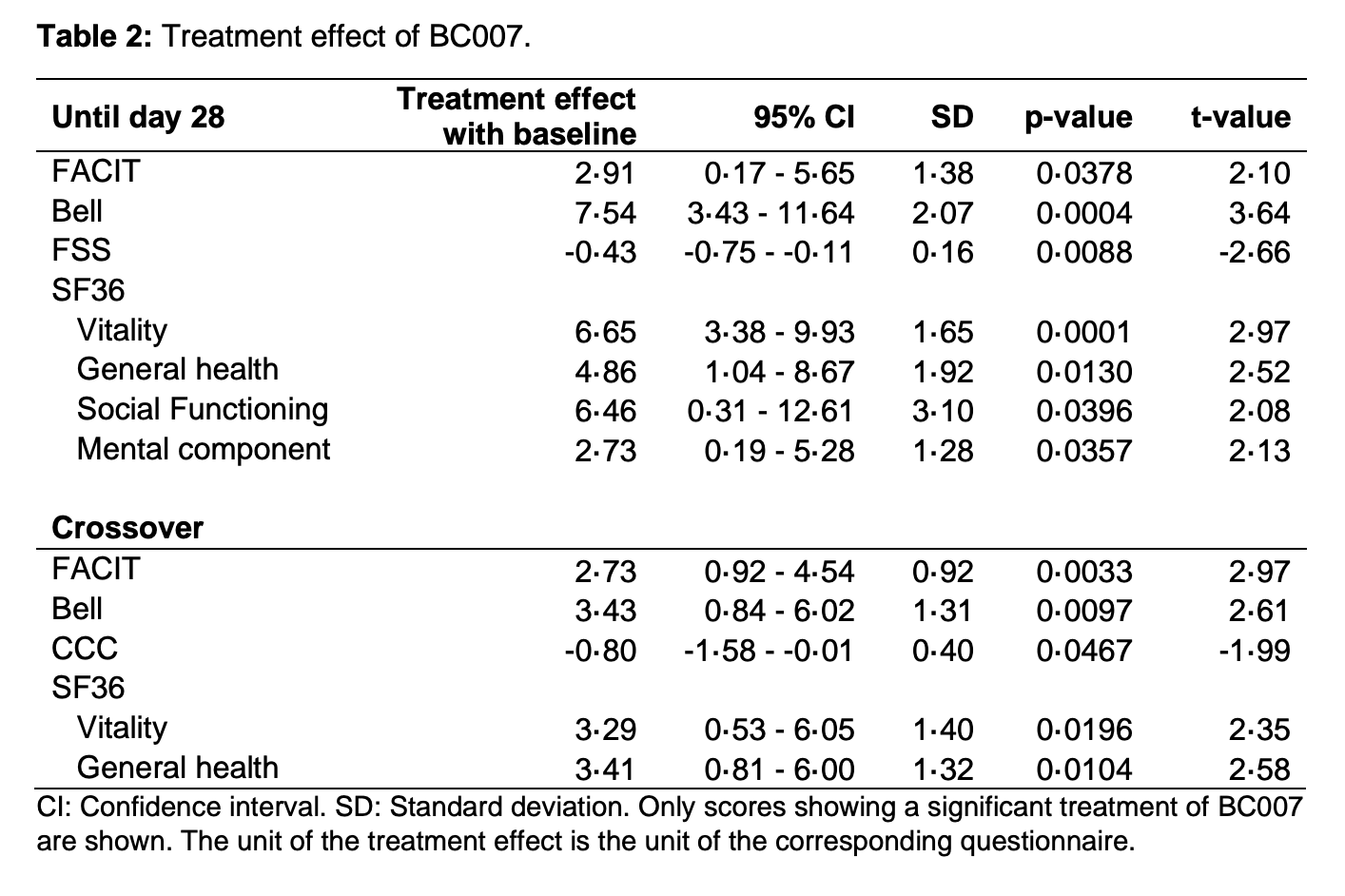
Findings: Between 31.10.2023 and 12.06.2024, 30 PCS patients were randomised and analysed. The trial has been concluded. Summarizing all AE rates, no statistically significant differences between sequence A und sequence B were observed within day 28 and day 70. One report of a serious adverse event, not related to treatment, was recorded. As a secondary endpoint, BC007 showed a significant improvement on self-reported fatigue and its severity, as well as quality of life.
Interpretation: As BC007 was well tolerated and showed a significant improvement of fatigue and quality of life, it might offer a therapeutic option for an autoimmune subgroup of PCS patients.
Trial registration: EudraCT, number 2022-001781-35.
Funding: German Federal Ministry of Education and Research, German Research Foundation.
Link | PDF
Bettina Hohberger, Marion Ganslmayer, Thomas Harrer, Friedrich Kruse, Stefanie Maas, Tobias Borst, Ralph Heimke-Brinck, Andreas Stog, Thomas Knauer, Eva Ruehl, Victoria Zeisberg, Adam Skornia, Alexander Bartsch, Armin Stroebel, Monika Wytopil, Carolin Merkel, Sophia Hofmann, Katja G. Schmidt, Petra Lakatos, Julia Schottenhamml, Martin Herrmann, Christian Mardin, Juergen Rech
Abstract
Background: As recent data suggest an involvement of GPCR-fAAb in PCS pathogenesis, neutralisation of such GPCR-fAAbs by BC007 could improve PCS symptoms. The aim of the reCOVer trial was to investigate safety, tolerability and clinical effects of BC007 on fatigue, its severity and quality of life in PCS patients.
Methods: reCOVer is a prospective, exploratory, randomized, placebo-controlled, double-blind, crossover phase IIa clinical trial with 1350 mg BC007 at the University of Erlangen-Nuernberg, Germany. Eligible participants were 18-80 years with GPCR-fAAb, whose PCS symptoms persisted ≥3 months after PCR-confirmed COVID-19, with fatigue as the major symptom (Bell score ≤60) and at least three of eight defined PCS symptoms. Participants were randomly assigned (1:1) according to a crossover design to either receive BC007 (sequence A) or placebo (sequence B) at day 0 and day 48 with a follow-up of 28 days, respectively. A crossover design was chosen to increase patient adherence. Occurrence of treatment-emergent adverse events (TEAEs) in comparison between sequence A and B from d0 to d28 and d0 to d70 were the primary and co-primary endpoint, respectively.
Findings: Between 31.10.2023 and 12.06.2024, 30 PCS patients were randomised and analysed. The trial has been concluded. Summarizing all AE rates, no statistically significant differences between sequence A und sequence B were observed within day 28 and day 70. One report of a serious adverse event, not related to treatment, was recorded. As a secondary endpoint, BC007 showed a significant improvement on self-reported fatigue and its severity, as well as quality of life.
Interpretation: As BC007 was well tolerated and showed a significant improvement of fatigue and quality of life, it might offer a therapeutic option for an autoimmune subgroup of PCS patients.
Trial registration: EudraCT, number 2022-001781-35.
Funding: German Federal Ministry of Education and Research, German Research Foundation.
Link | PDF