Chandelier
Senior Member (Voting Rights)
Rapid amyloid-β clearance and cognitive recovery through multivalent modulation of blood–brain barrier transport - Signal Transduction and Targeted Therapy
The blood‒brain barrier (BBB) is a highly selective permeability barrier that safeguards the central nervous system (CNS) from potentially harmful substances while regulating the transport of essential molecules. Its dysfunction is increasingly recognized as a pivotal factor in the pathogenesis...
Abstract
The blood‒brain barrier (BBB) is a highly selective permeability barrier that safeguards the central nervous system (CNS) from potentially harmful substances while regulating the transport of essential molecules.Its dysfunction is increasingly recognized as a pivotal factor in the pathogenesis of Alzheimer’s disease (AD), contributing to the accumulation of amyloid-β (Aβ) plaques.
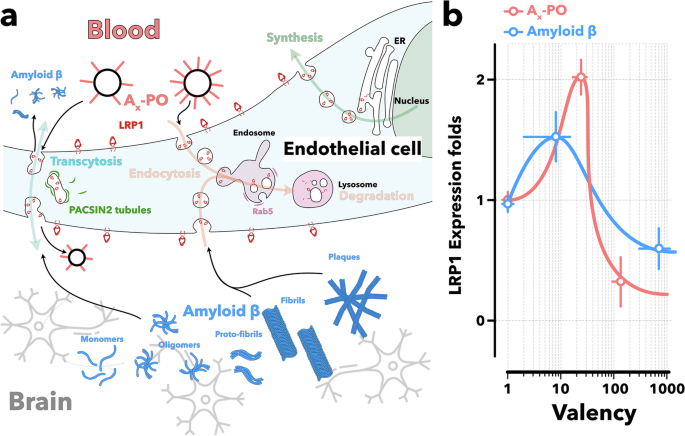
We present a novel therapeutic strategy that targets low-density lipoprotein receptor-related protein 1 (LRP1) on the BBB.
Our design leverages the multivalent nature and precise size of LRP1-targeted polymersomes to modulate receptor-mediated transport, biasing LRP1 trafficking toward transcytosis and thereby upregulating its expression to promote efficient Aβ removal. In AD model mice, this intervention significantly reduced brain Aβ levels by nearly 45% and increased plasma Aβ levels by 8-fold within 2 h, as measured by ELISA. Multiple imaging techniques confirmed the reduction in brain Aβ signals after treatment.
Cognitive assessments revealed that treated AD mice exhibited significant improvements in spatial learning and memory, with performance levels comparable to those of wild-type mice.
These cognitive benefits persisted for up to 6 months post-treatment.
This work pioneers a new paradigm in drug design, where function arises from the supramolecular nature of the nanomedicine, harnessing multivalency to elicit biological action at the membrane trafficking level.
Our findings also reaffirm the critical role of the BBB in AD pathogenesis and demonstrate that targeting the BBB can make therapeutic interventions significantly more effective.
We establish a compelling case for BBB modulation and LRP1-mediated Aβ clearance as a transformative foundation for future AD therapies.