Prolonged platelet hyperactivity after COVID-19 infection
Noriko Nara; Mie Shimizu; Masahiro Yamamoto; Tomoki Nakamizo; Azusa Hayakawa; Ken Johkura
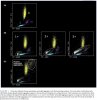
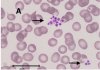
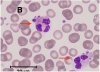
Platelet hyperactivity often occurs in patients with coronavirus disease 2019 (COVID-19). However, it remains unclear how long platelet hyperactivity lasts after the acute phase, owing to a lack of follow-up studies. To elucidate the course of platelet hyperactivity, we serially measured platelet activity in patients with COVID-19 up to 40 days after hospital admission using an easily assessable haematology analyser that semi-quantitates platelet clumps on a scattergram.
Our results showed that platelet hyperactivity persisted for at least 40 days even after acute inflammation subsided in most patients with COVID-19, regardless of disease severity. Persistent platelet hyperactivity may contribute to thromboembolic complications in post-COVID-19 patients.
Link | Paywall (British Journal of Haematology)
Noriko Nara; Mie Shimizu; Masahiro Yamamoto; Tomoki Nakamizo; Azusa Hayakawa; Ken Johkura
Platelet hyperactivity often occurs in patients with coronavirus disease 2019 (COVID-19). However, it remains unclear how long platelet hyperactivity lasts after the acute phase, owing to a lack of follow-up studies. To elucidate the course of platelet hyperactivity, we serially measured platelet activity in patients with COVID-19 up to 40 days after hospital admission using an easily assessable haematology analyser that semi-quantitates platelet clumps on a scattergram.
Our results showed that platelet hyperactivity persisted for at least 40 days even after acute inflammation subsided in most patients with COVID-19, regardless of disease severity. Persistent platelet hyperactivity may contribute to thromboembolic complications in post-COVID-19 patients.
Link | Paywall (British Journal of Haematology)