Dolphin
Senior Member (Voting Rights)
Nutraceutical Supplementation Effects on Subjective Fatigue Symptoms in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating condition marked by severe, long-lasting fatigue and exhaustion that does not improve with rest. ME/CFS is reported in individuals of all ages and various racial, socioeconomic, and ethnic groups. This condition lacks...
Nutraceutical Supplementation Effects on Subjective Fatigue Symptoms in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review
Emanuella M. Brito • Leonardo Bonifanti • Rajvi Patel • Jailene Jimenez • Jacqueline Junco • Irina R. Rozenfeld • Violetta Renesca • Amanpreet K. Cheema
Published: July 02, 2025
DOI: 10.7759/cureus.87178
Peer-Reviewed
Cite this article as: Brito E M, Bonifanti L, Patel R, et al. (July 02, 2025) Nutraceutical Supplementation Effects on Subjective Fatigue Symptoms in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review. Cureus 17(7): e87178. doi:10.7759/cureus.87178
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating condition marked by severe, long-lasting fatigue and exhaustion that does not improve with rest. ME/CFS is reported in individuals of all ages and various racial, socioeconomic, and ethnic groups.This condition lacks standard treatment. Nutritional supplements and dietary interventions are often used to manage symptoms, but the efficacy of these interventions remains scarce in the current literature.
This systematic review aims to evaluate and summarize recent evidence on nutrient supplementation and diet-based interventions in patients with ME/CFS sourced from clinical trial registries and article databases.
Registries improve the quality, integrity, and transparency of clinical trials by providing a standardized platform for reporting study design and results and, thus, reducing the biases related to selective reporting practices.
Systematic reviews using these registries, therefore, are an efficient pathway to acquire current medical evidence for use in clinical decision-making and the development of practice guidance in various fields.
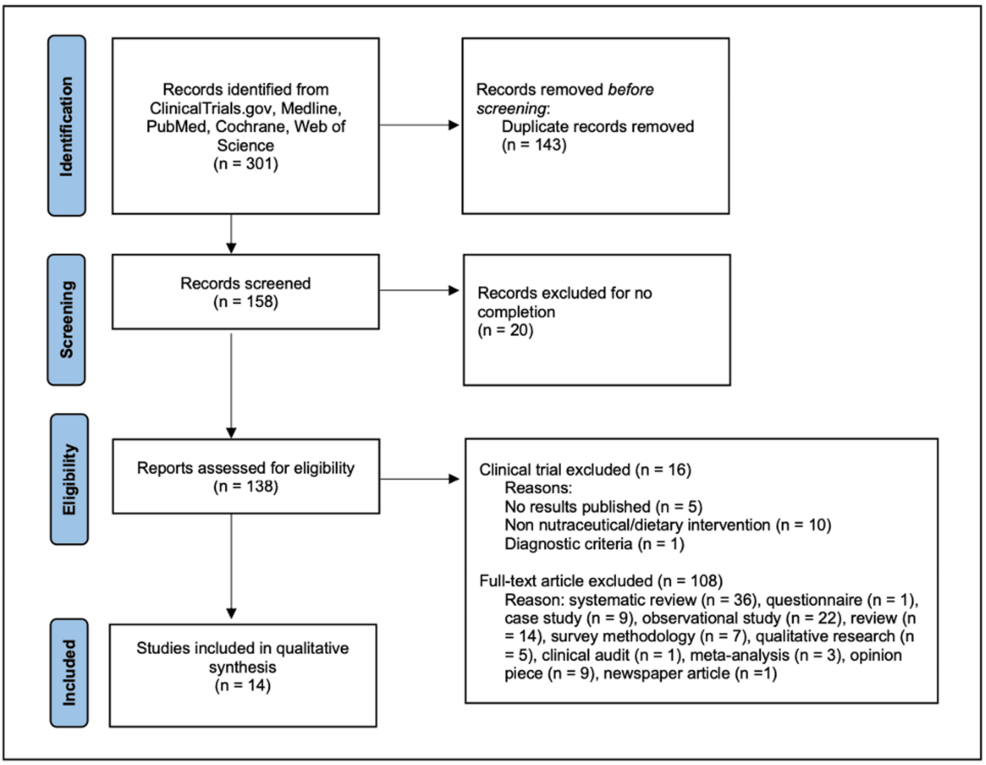
ClinicalTrials.gov, Medline, PubMed, Cochrane, and Web of Science were systematically searched for interventional studies in which patients suffering from ME/CFS supplemented or altered their diet.
The results of this review showed several supplements that suggest improvement in patients’ symptomatology, including nicotinamide adenine dinucleotide (NADH), coenzyme Q10 (CoQ10), wasabi, and probiotics.
However, many of these registered clinical trials did not employ the U.S. National Institutes of Health (NIH)’s National Institute of Neurological Disorders and Stroke (NINDS) suggested common data elements (CDEs).
These standardized outcome-measuring tools allow the generalization and true comparison of the patient-reported outcomes.
Last edited:
