Myopathy as a cause of Long COVID fatigue: Evidence from quantitative and single fiber EMG and muscle histopathology
Highlights
To describe neurophysiological abnormalities in Long COVID and correlate quantitative electromyography (qEMG) and single fiber EMG (sfEMG) results to clinical scores and histopathology.
Methods
84 patients with non-improving musculoskeletal Long COVID symptoms were examined with qEMG and sfEMG. Muscle biopsies were taken in a subgroup.
Results
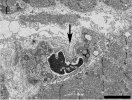
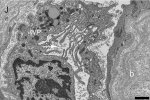
Mean motor unit potential (MUP) duration was decreased in ≥ 1 muscles in 52 % of the patients. Mean jitter was increased in 17 % of the patients in tibialis anterior and 25 % in extensor digitorum communis. Increased jitter was seen with or without myopathic qEMG. Low quality of life score correlated with higher jitter values but not with qEMG measures. In addition to our previously published mitochondrial changes, inflammation, and capillary injury, we show now in muscle biopsies damage of terminal nerves and motor endplate with abundant basal lamina material. At the endplate, axons were present but no vesicle containing terminals. The post-synaptic cleft in areas appeared atrophic with short clefts and coarse crests.
Conclusions
Myopathic changes are common in Long COVID. sfEMG abnormality is less common but may correlate with clinical scores. sfEMG changes may be due to motor endplate pathology.
https://www.sciencedirect.com/science/article/pii/S1388245723000196#b0040
This seems to build on similar work discussed here: https://www.s4me.info/threads/myopa...uscle-histopathology-2022-hejbøl-et-al.27997/.
Highlights
- Myopathic changes in qEMG and/or increased jitter in sfEMG were seen in 63% of 84 patients with Long COVID neuromuscularsymptoms.
- Low quality of life score correlated with higher mean jitter values in sfEMG but not with qEMG measures.
- Electron microscopy showed damage of terminal nerves and motor endplate.
To describe neurophysiological abnormalities in Long COVID and correlate quantitative electromyography (qEMG) and single fiber EMG (sfEMG) results to clinical scores and histopathology.
Methods
84 patients with non-improving musculoskeletal Long COVID symptoms were examined with qEMG and sfEMG. Muscle biopsies were taken in a subgroup.
Results
Mean motor unit potential (MUP) duration was decreased in ≥ 1 muscles in 52 % of the patients. Mean jitter was increased in 17 % of the patients in tibialis anterior and 25 % in extensor digitorum communis. Increased jitter was seen with or without myopathic qEMG. Low quality of life score correlated with higher jitter values but not with qEMG measures. In addition to our previously published mitochondrial changes, inflammation, and capillary injury, we show now in muscle biopsies damage of terminal nerves and motor endplate with abundant basal lamina material. At the endplate, axons were present but no vesicle containing terminals. The post-synaptic cleft in areas appeared atrophic with short clefts and coarse crests.
Conclusions
Myopathic changes are common in Long COVID. sfEMG abnormality is less common but may correlate with clinical scores. sfEMG changes may be due to motor endplate pathology.
https://www.sciencedirect.com/science/article/pii/S1388245723000196#b0040
This seems to build on similar work discussed here: https://www.s4me.info/threads/myopa...uscle-histopathology-2022-hejbøl-et-al.27997/.