Sly Saint
Senior Member (Voting Rights)
Abstract
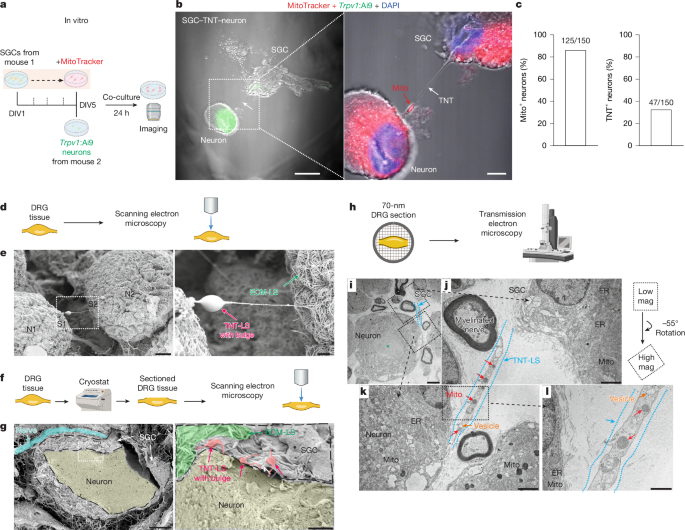
Primary sensory neurons in dorsal root ganglia (DRG) have long axons and a high demand for mitochondria, and mitochondrial dysfunction has been implicated in peripheral neuropathy after diabetes and chemotherapy1,2. However, the mechanisms by which primary sensory neurons maintain their mitochondrial supply remain unclear. Satellite glial cells (SGCs) in DRG encircle sensory neurons and regulate neuronal activity and pain3. Here we show that SGCs are capable of transferring mitochondria to DRG sensory neurons in vitro, ex vivo and in vivo by the formation of tunnelling nanotubes with SGC-derived myosin 10 (MYO10). Scanning and transmission electron microscopy revealed tunnelling nanotube-like ultrastructures between SGCs and sensory neurons in mouse and human DRG. Blockade of mitochondrial transfer in naive mice leads to nerve degeneration and neuropathic pain. Single-nucleus RNA sequencing and in situ hybridization revealed that MYO10 is highly expressed in human SGCs. Furthermore, SGCs from DRG of people with diabetes exhibit reduced MYO10 expression and mitochondrial transfer to neurons. Adoptive transfer of human SGCs into the mouse DRG provides MYO10-dependent protection against peripheral neuropathy. This study uncovers a previously unrecognized role of peripheral glia and provides insights into small fibre neuropathy in diabetes, offering new therapeutic strategies for the management of neuropathic pain.
 www.nature.com
www.nature.com
see also
Replenishing mitochondria significantly reduces chronic nerve pain, research shows
 www.news-medical.net
www.news-medical.net
Primary sensory neurons in dorsal root ganglia (DRG) have long axons and a high demand for mitochondria, and mitochondrial dysfunction has been implicated in peripheral neuropathy after diabetes and chemotherapy1,2. However, the mechanisms by which primary sensory neurons maintain their mitochondrial supply remain unclear. Satellite glial cells (SGCs) in DRG encircle sensory neurons and regulate neuronal activity and pain3. Here we show that SGCs are capable of transferring mitochondria to DRG sensory neurons in vitro, ex vivo and in vivo by the formation of tunnelling nanotubes with SGC-derived myosin 10 (MYO10). Scanning and transmission electron microscopy revealed tunnelling nanotube-like ultrastructures between SGCs and sensory neurons in mouse and human DRG. Blockade of mitochondrial transfer in naive mice leads to nerve degeneration and neuropathic pain. Single-nucleus RNA sequencing and in situ hybridization revealed that MYO10 is highly expressed in human SGCs. Furthermore, SGCs from DRG of people with diabetes exhibit reduced MYO10 expression and mitochondrial transfer to neurons. Adoptive transfer of human SGCs into the mouse DRG provides MYO10-dependent protection against peripheral neuropathy. This study uncovers a previously unrecognized role of peripheral glia and provides insights into small fibre neuropathy in diabetes, offering new therapeutic strategies for the management of neuropathic pain.

Mitochondrial transfer from glia to neurons protects against peripheral neuropathy - Nature
Mitochondria that are transported from satellite glial cells in dorsal root ganglia to peripheral sensory neurons through tunneling nanotube-like structures provide protection against peripheral neuropathy.
see also
Replenishing mitochondria significantly reduces chronic nerve pain, research shows
For millions living with nerve pain, even a light touch can feel unbearable. Scientists have long suspected that damaged nerve cells falter because their energy factories known as mitochondria don't function properly.
Now research published in Nature suggests a way forward: supplying healthy mitochondria to struggling nerve cells.
Using human tissue and mouse models, researchers at Duke University School of Medicine found that replenishing mitochondria significantly reduced pain tied to diabetic neuropathy and chemotherapy-induced nerve damage. In some cases, the relief lasted up to 48 hours.
Instead of masking symptoms, the approach could fix what the team sees as the root problem - restoring the energy flow that keeps nerve cells healthy and resilient.

Replenishing mitochondria significantly reduces chronic nerve pain, research shows
For millions living with nerve pain, even a light touch can feel unbearable. Scientists have long suspected that damaged nerve cells falter because their energy factories known as mitochondria don't function properly.
