Chandelier
Senior Member (Voting Rights)
Authors:
Published: 25 June 2025
DOI: https://doi.org/10.1038/s41541-025-01172-3
 www.nature.com
www.nature.com
- Maxime Taquet
- John A. Todd
- Paul J. Harrison
Abstract
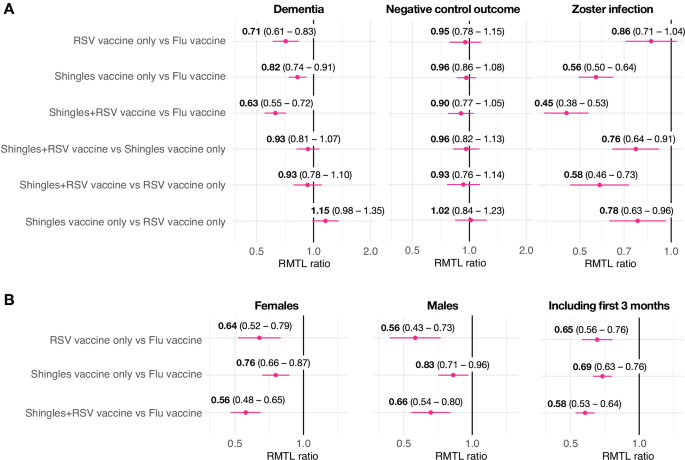
AS01-adjuvanted shingles (herpes zoster) vaccination is associated with a lower risk of dementia, but the underlying mechanisms are unclear. In propensity-score matched cohort studies with 436,788 individuals, both the AS01-adjuvanted shingles and respiratory syncytial virus (RSV) vaccines, individually or combined, were associated with reduced 18-month risk of dementia. No difference was observed between the two AS01-adjuvanted vaccines, suggesting that the AS01 adjuvant itself plays a direct role in lowering dementia risk.Published: 25 June 2025
DOI: https://doi.org/10.1038/s41541-025-01172-3

Lower risk of dementia with AS01-adjuvanted vaccination against shingles and respiratory syncytial virus infections - npj Vaccines
AS01-adjuvanted shingles (herpes zoster) vaccination is associated with a lower risk of dementia, but the underlying mechanisms are unclear. In propensity-score matched cohort studies with 436,788 individuals, both the AS01-adjuvanted shingles and respiratory syncytial virus (RSV) vaccines...
Last edited by a moderator:
