Dolphin
Senior Member (Voting Rights)
Individualized online exercise therapy aids recovery in pediatric long-COVID - Findings from an exploratory randomized controlled trial
Purpose To evaluate the feasibility, safety, and effectiveness of an individualized online exercise therapy (IOET) designed to improve physical capacity and quality of life in children and adolescents with long-COVID. Methods In this prosp...
Individualized online exercise therapy aids recovery in pediatric long-COVID - Findings from an exploratory randomized controlled trial
Sarah Christina Goretzki1Mara Bergelt2
Laurent Weis2
Rayan Hojeii1
Gabriele Gauß1
Miriam Götte1
Ronja Beller1
Sven Benson Prof3
Anne Schönecker1
Adela Della Marina1
Andrea Gangfuß1
Florian Stehling1
Christina Pentek1
Anna von Loewenich1
Tom Hühne1
Clara Held1
Sebastian Voigt4
Ursula Felderhoff-Müser1
Michael M. Schündeln1
Nora Bruns1
Katharina Eckert Prof2
Christian Dohna-Schwake1
Maire Brasseler1
1 University Duisburg-Essen, Children’s Hospital Essen, 45147 Essen, Germany,
2 IST University of Applied Sciences, Health Management and Public Health, Düsseldorf, Germany,
3 University Hospital Essen, University of Duisburg-Essen, Essen, Germany,
4 University Hospital Essen, University of Duisburg-Essen, 45147 Essen, Germany
This is a preprint; it has not been peer reviewed by a journal.
Individualized online exercise therapy aids recovery in pediatric long-COVID - Findings from an exploratory randomized controlled trial
Purpose To evaluate the feasibility, safety, and effectiveness of an individualized online exercise therapy (IOET) designed to improve physical capacity and quality of life in children and adolescents with long-COVID. Methods In this prosp...
This work is licensed under a CC BY 4.0 License
Abstract
Purpose
To evaluate the feasibility, safety, and effectiveness of an individualized online exercise therapy (IOET) designed to improve physical capacity and quality of life in children and adolescents with long-COVID.
Methods
In this prospective, randomized, single-centre controlled trial, 14 patients aged 9–17 years (11 females, 3 males) with long-COVID (median symptom duration: 21 months) were assigned to either 6 or 12 weeks of IOET. The intervention comprised twice-weekly, telemedicine-delivered sessions tailored to individual ability and symptom burden, supplemented with diaries, activity trackers, and handouts for self-studies. Primary outcomes were physical performance (6-Minute Walk Test [6MWT], Sit-to-Stand Test [STST], Handgrip Strength Test [HST]). Secondary outcomes included school attendance, quality of life (PedsQL), physical activity, safety, and self-reported recovery.
Results
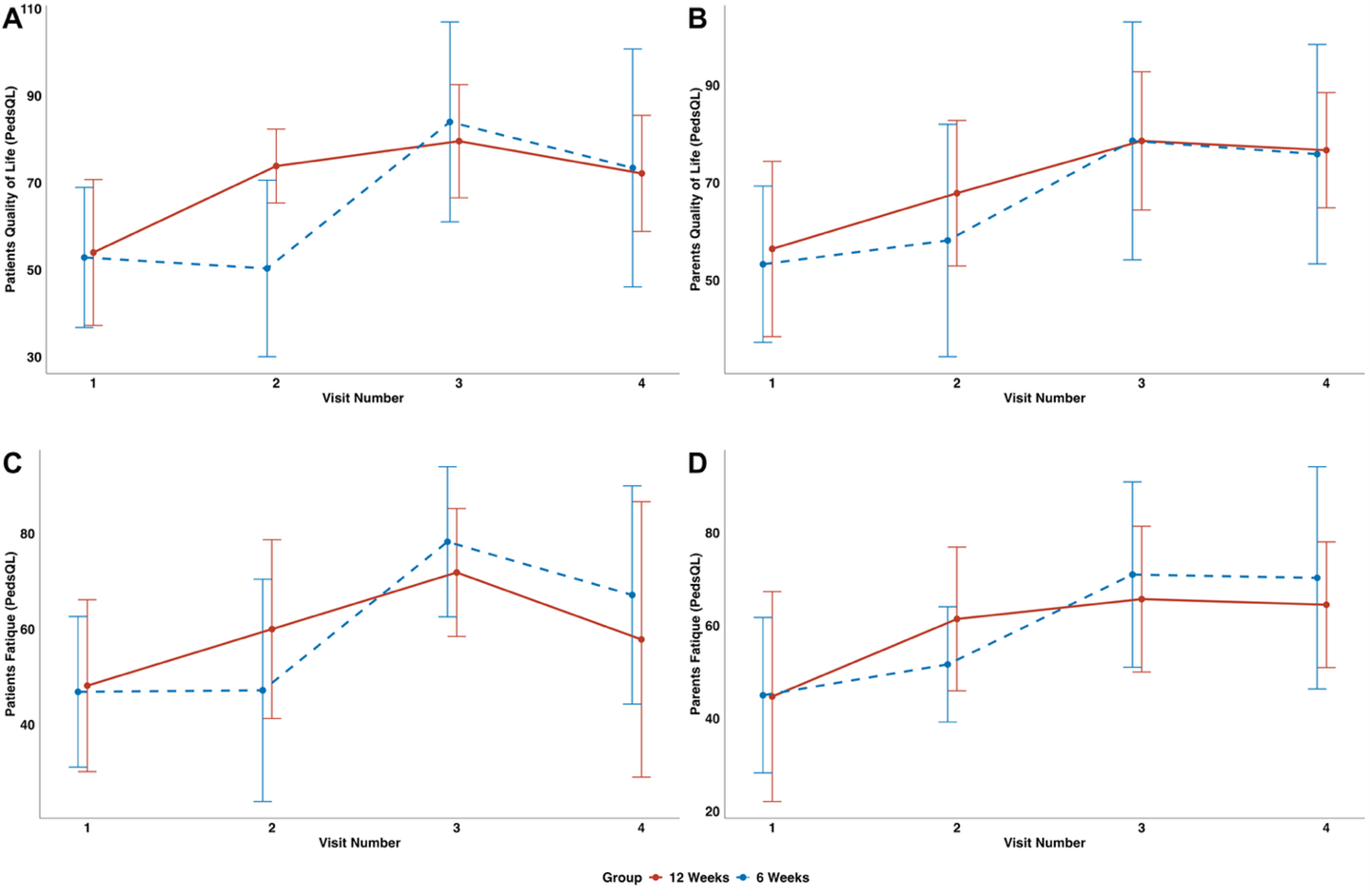
All participants showed clinically relevant improvements. In the 12-week IOET group, 6MWT increased from 396.0 m to 616.3 m, STST from 25.4 to 32.6 repetitions, and HST from 16.6 kg to 27.1 kg. The 6-week group improved comparably (6MWT: 429.0 m to 601.6 m; STST: 21.6 to 31.7; HST: 17.3 to 22.1 kg). School attendance rose from 58% to 97%, and PedsQL scores reflected improved quality of life and reduced fatigue. No adverse events or post-exertional symptom exacerbations occurred. Improvements persisted at 3-month follow-up, although some decline from peak performance was noted.
Conclusions
IOET is feasible, safe, and associated with improved physical function, reintegration in every day life and it’s quality in pediatric long-COVID. These findings highlight IOET as a promising rehabilitation strategy and justify larger multicentre trials to confirm effectiveness and define optimal programme duration.
Pediatric long-COVID
exercise therapy
fatigue
physical health
mental health
school participation