Chandelier
Senior Member (Voting Rights)
High sensitivity perovskite-tagged nanobody electrochemiluminescent immunosensor for spike (S1) protein biomarker-based persistent viral reservoir detection
Several studies have identified spike protein (S1) as a definitive biomarker for the early detection of persistent viral reservoirs and latent COVID-19.
A novel sandwich-format electrochemical immunosensor integrating a nanocomposite material was engineered for rapid and sensitive latent COVID-19 detection.
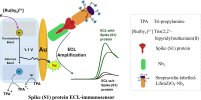
The platform structure, AuSPE||strep|Nb1|BSA|biotin|S1|strep-LiSmZrO3-Nb2 + BSA (AuSPE = gold screen-printed electrode, strep = streptavidin-thiol, Nb1 = primary nanobody, Nb2 = secondary nanobody, BSA = bovine serum albumin, and spike (S1) protein = S1), featured a disposable AuSPE modified with strep to anchor a biotinylated camelid Nb1 specific to the spike protein.
A Nb2 conjugated to streptavidin-labelled LiSmZrO3 perovskite completed the sandwich complex, enhancing both affinity and signal transduction.
Electrochemical responses of the sensor were studied via electrochemiluminescent (ECL) signal transduction.
The S1-sensitive sandwich immunosensor had a detection range of 0–1000 pg mL−1 with a limit of detection of 0.04 pg mL−1 via ECL.
As feasibility studies, commercial spike protein in buffered solutions and human serum, highlighting the potential for the immunosensor to be used in SARS-CoV-2 patients and PVRs.
The nanobody sandwich immunosensor showed excellent stability, selectivity, sensitivity, and reproducibility.
The immunosensor serves as a broad PVR for a SARS-CoV-2 screening tool, detecting elevated S1 levels to enable early, targeted diagnostics.
Web | DOI | Bioelectrochemistry
January, Jaymi; Zwaenepoel, Olivier; Sanga, Nelia; Gettemans, Jan; Iwuoha, Emmanuel
Highlights
- First perovskite-tagged nanobody ECL sensor for SARS-CoV-2S1 detection.
- Ultra-sensitive detection with high specificity and robust stability.
- Achieves very low detection limits in biological samples.
- Rapid, reliable platform for COVID-19 protein detection.
- Enables non-invasive monitoring of long-term viral persistence.
Abstract
Persistent viral reservoirs (PVR) of SARS-CoV-2 (or long COVID-19) and latent COVID-19 disease have been of great concern to clinicians.Several studies have identified spike protein (S1) as a definitive biomarker for the early detection of persistent viral reservoirs and latent COVID-19.
A novel sandwich-format electrochemical immunosensor integrating a nanocomposite material was engineered for rapid and sensitive latent COVID-19 detection.
The platform structure, AuSPE||strep|Nb1|BSA|biotin|S1|strep-LiSmZrO3-Nb2 + BSA (AuSPE = gold screen-printed electrode, strep = streptavidin-thiol, Nb1 = primary nanobody, Nb2 = secondary nanobody, BSA = bovine serum albumin, and spike (S1) protein = S1), featured a disposable AuSPE modified with strep to anchor a biotinylated camelid Nb1 specific to the spike protein.
A Nb2 conjugated to streptavidin-labelled LiSmZrO3 perovskite completed the sandwich complex, enhancing both affinity and signal transduction.
Electrochemical responses of the sensor were studied via electrochemiluminescent (ECL) signal transduction.
The S1-sensitive sandwich immunosensor had a detection range of 0–1000 pg mL−1 with a limit of detection of 0.04 pg mL−1 via ECL.
As feasibility studies, commercial spike protein in buffered solutions and human serum, highlighting the potential for the immunosensor to be used in SARS-CoV-2 patients and PVRs.
The nanobody sandwich immunosensor showed excellent stability, selectivity, sensitivity, and reproducibility.
The immunosensor serves as a broad PVR for a SARS-CoV-2 screening tool, detecting elevated S1 levels to enable early, targeted diagnostics.
Graphical abstract

Web | DOI | Bioelectrochemistry
