Digitally Assessed Long COVID Symptomatology Is Associated With Lymphocyte Mitochondrial Dysfunction and Altered Immune Potential
BACKGROUND
Postacute sequelae of SARS-CoV-2 infection, also known as long COVID (LC), is a complex and heterogenous condition affecting millions worldwide with a poorly understood underlying pathology. Although metabolic dysregulations have been described in LC, it remains unclear whether circulating immune cells exhibit immunometabolic alterations.
METHODS
We conducted a detailed clinical, immunologic, and mitochondrial analysis on 27 patients with LC and 27 who recovered from COVID-19 and were healthy. Symptom burden and severity were assessed and quantified via a digital platform with the modified COVID-19 Yorkshire Rehabilitation Scale. Mitochondrial function of circulating immune cell populations (lymphocytes and monocytes) was analyzed by measuring mitochondrial mass and mitochondrial membrane potential. Production of 11 cytokines after whole blood stimulation with bacterial and viral agonists was measured by multiplex immunoassay. Relationships between mitochondrial and immune parameters with LC symptoms were investigated.
RESULTS
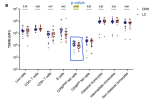
Patients with LC exhibited significant symptom burden, with worsening across all symptom domains as compared with their health state before SARS-CoV-2 infection. They also had a decreased mitochondrial membrane potential of CD56bright natural killer cells, particularly in patients experiencing dizziness, whereas reduced mitochondrial membrane potential in CD4+ lymphocytes was found in patients with worsening breathlessness. Upon LPS stimulation, patients with LC demonstrated significantly lower IFN-γ production. In response to viral ligand R848, impaired IFN-β and IL-10 responses were associated with worsening cough and executive functions.
CONCLUSIONS
Symptom severity in LC is associated with immune cell mitochondrial dysfunction and altered cytokine responses, highlighting potential disease biomarkers and targets for future therapeutic strategies.
Web | DOI | PDF | Open Forum Infectious Diseases | Open Access
Sularea, Vasile Mihai; Townsend, Liam; Reid, Cian; Atanasescu, Andreea V; Dyer, Adam H; Giangrazi, Federica; Lawrence, Roman Rocha; Sivan, Manoj; Moran, Barry; Doherty, Derek G; Conlon, Niall P; Long, Aideen; O’Farrelly, Cliona
BACKGROUND
Postacute sequelae of SARS-CoV-2 infection, also known as long COVID (LC), is a complex and heterogenous condition affecting millions worldwide with a poorly understood underlying pathology. Although metabolic dysregulations have been described in LC, it remains unclear whether circulating immune cells exhibit immunometabolic alterations.
METHODS
We conducted a detailed clinical, immunologic, and mitochondrial analysis on 27 patients with LC and 27 who recovered from COVID-19 and were healthy. Symptom burden and severity were assessed and quantified via a digital platform with the modified COVID-19 Yorkshire Rehabilitation Scale. Mitochondrial function of circulating immune cell populations (lymphocytes and monocytes) was analyzed by measuring mitochondrial mass and mitochondrial membrane potential. Production of 11 cytokines after whole blood stimulation with bacterial and viral agonists was measured by multiplex immunoassay. Relationships between mitochondrial and immune parameters with LC symptoms were investigated.
RESULTS
Patients with LC exhibited significant symptom burden, with worsening across all symptom domains as compared with their health state before SARS-CoV-2 infection. They also had a decreased mitochondrial membrane potential of CD56bright natural killer cells, particularly in patients experiencing dizziness, whereas reduced mitochondrial membrane potential in CD4+ lymphocytes was found in patients with worsening breathlessness. Upon LPS stimulation, patients with LC demonstrated significantly lower IFN-γ production. In response to viral ligand R848, impaired IFN-β and IL-10 responses were associated with worsening cough and executive functions.
CONCLUSIONS
Symptom severity in LC is associated with immune cell mitochondrial dysfunction and altered cytokine responses, highlighting potential disease biomarkers and targets for future therapeutic strategies.
Web | DOI | PDF | Open Forum Infectious Diseases | Open Access