Chandelier
Senior Member (Voting Rights)
A parabrachial hub for need-state control of enduring pain - Nature
Activity in a set of parabranchial neurons in the mouse brain is increased during chronic pain, predicts coping behaviour, and can be modulated by circuits activated by survival threats.
- Nitsan Goldstein,
- Amadeus Maes,
- Heather N. Allen,
- Tyler S. Nelson,
- Kayla A. Kruger,
- Morgan Kindel,
- Albert T. M. Yeung,
- Nicholas K. Smith,
- Jamie R. E. Carty,
- Lavinia Boccia,
- Niklas Blank,
- Emily Lo,
- Rachael E. Villari,
- Ella Cho,
- Erin L. Marble,
- Michelle Awh,
- Yasmina Dumiaty,
- Melissa J. Chee,
- Rajesh Khanna,
- Christoph A. Thaiss,
- Bradley K. Taylor,
- Ann Kennedy &
- J. Nicholas Betley
Abstract
Long-term sustained pain following acute physical injury is a prominent feature of chronic pain conditions1.Populations of neurons that rapidly respond to noxious stimuli or tissue damage have been identified in the spinal cord and several nuclei in the brain2–4.
Understanding the central mechanisms that signal ongoing sustained pain, including after tissue healing, remains a challenge5.
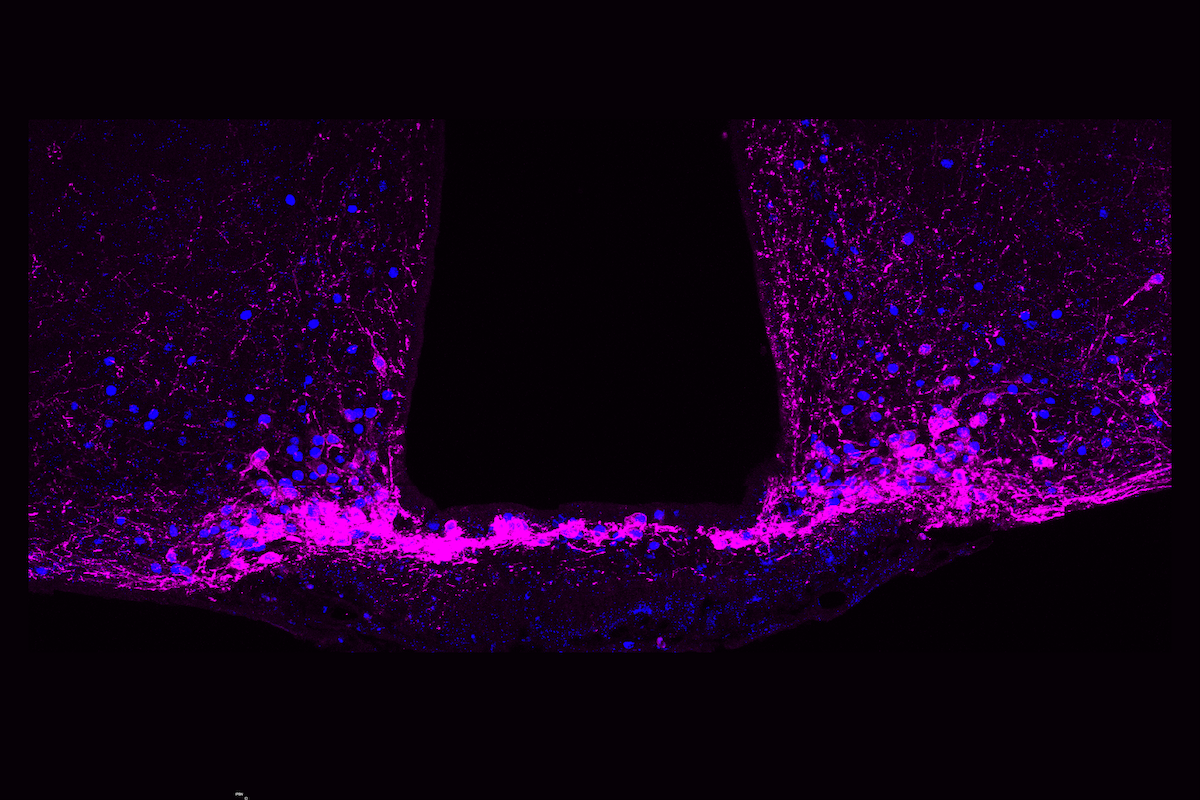
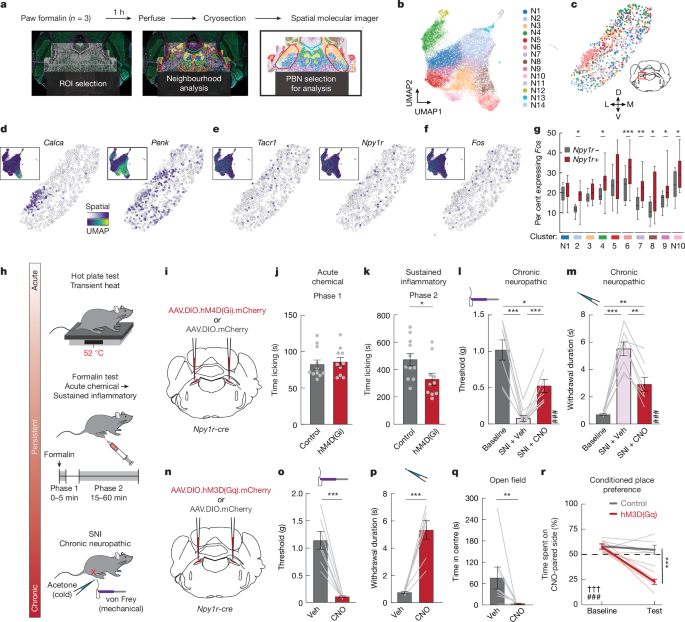
Here we use spatial transcriptomics, neural manipulations, activity recordings and computational modelling to demonstrate that activity in an ensemble of anatomically and molecularly diverse parabrachial neurons that express the neuropeptide Y (NPY) receptor Y1 (Y1R neurons) is increased following injury and predicts functional coping behaviour.
Hunger, thirst or predator cues suppressed sustained pain, regardless of the injury type, by inhibiting parabrachial Y1R neurons via the release of NPY. Together, our results demonstrate an endogenous analgesic hub at pain-responsive parabrachial Y1R neurons.